BATCH TRACKING FOR MEDICAL & LAB SUPPLIES
Expiry Management for Medical & Lab Supplies — Audit-Ready, Error-Free
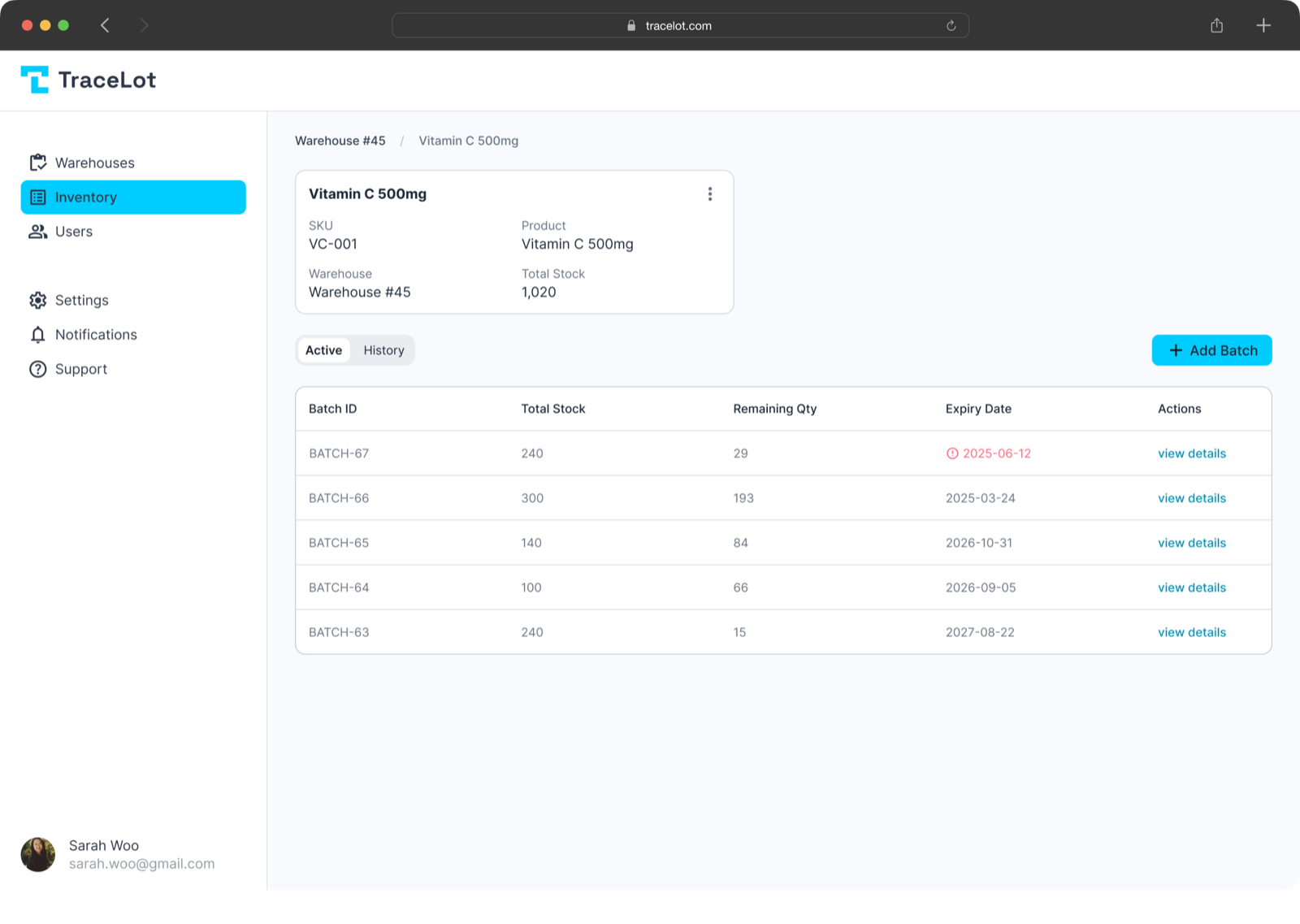
TraceLot automates FEFO allocation, blocks expired inventory, and maintains the lot-level traceability FDA and ISO standards require — fully synced with Veeqo.
First month free. Cancel anytime.
The Problem
Why Medical & Lab Supply Distributors Need Automated Batch Tracking
Pain Point
Critical Expiry Accuracy
Expired reagents produce invalid test results. Expired medical supplies create patient safety risks. In medical and lab contexts, expiry accuracy isn't optional — it's critical.
Pain Point
High-Value Inventory Write-Offs
Reagents, test kits, and medical supplies are expensive. Without FEFO automation, newer stock ships while older batches expire — destroying significant inventory value.
Pain Point
FDA and ISO Documentation Requirements
Medical device regulations and ISO 13485 require documented batch traceability. GLP and CLIA standards mandate reagent lot tracking. Spreadsheets can't meet these requirements reliably.
Pain Point
Lot Recall Complexity
When a manufacturer issues a lot recall, can you identify every lab or facility that received affected products immediately? Manual tracking delays critical safety responses.
The Solution
How TraceLot Protects Medical & Lab Supply Operations
Solution
Automatic FEFO on Every Order
Every order automatically receives the earliest-expiring batch. Your team sees exact lot numbers in Veeqo — ensuring labs and facilities receive products with maximum remaining shelf life.
Solution
Expiry Firewall
Expired medical supplies and reagents are automatically blocked from Veeqo inventory. No expired products can be sold, protecting patient safety and test validity.
Solution
FDA & ISO-Ready Traceability
Maintain complete lot-level records for FDA medical device requirements, ISO 13485, and laboratory standards. Full batch history from manufacturer through customer.
Solution
Instant Recall Capability
Generate a complete list of affected customers by manufacturer lot number in minutes. Respond to FDA recalls and safety alerts immediately.
Ready to streamline your medical & lab supplies inventory?
Join hundreds of medical & lab supplies businesses already using TraceLot to eliminate expired shipments and stay compliant.
Regulatory Compliance
Built for Medical & Laboratory Standards
Medical devices, lab reagents, and supplies face rigorous regulatory requirements. TraceLot provides compliant batch traceability infrastructure.
Regulation
FDA Medical Device (21 CFR Part 820)
Quality System Regulation requires documented batch control and distribution records.
- Lot-level traceability
- Distribution records
- Expiry documentation
- Recall response capability
Regulation
ISO 13485 Quality Management
Medical device quality standards require documented batch tracking. TraceLot helps maintain the traceability records needed.
- Batch identification
- Traceability records
- Distribution documentation
- Audit-ready batch history
Regulation
Laboratory Standards (GLP, CLIA, CAP)
Laboratory regulations require reagent lot tracking and expiry management.
- Reagent lot documentation
- Expiry tracking
- Calibrator/control lot records
- Chain of custody
Results Medical & Lab Distributors Achieve with TraceLot
0
Expired products shipped
100%
Lot traceability
<5 min
Recall report generation
Audit
Ready at all times
How It Works
Get Started in 10 Minutes
1
Connect Veeqo
Link your Veeqo account with OAuth in 2 minutes. No code, no IT support needed.
2
Add Batches
Add batches with manufacturer lot numbers and expiry dates. Track calibration dates, storage requirements, and certification information as needed.
3
Ship with Confidence
TraceLot auto-assigns FEFO batches to every order. Expired products are blocked automatically. Labs receive products with maximum usable life.
Comparison
Manual Tracking vs TraceLot
| Capability | Manual Tracking | With TraceLot |
|---|---|---|
| Batch Tracking | Error-prone spreadsheets | Automated batch assignment |
| FEFO Allocation | Manual picker instructions | Automatic on every order |
| Expired Stock Protection | Risk of shipping expired medical and lab products | Expiry Firewall blocks automatically |
| FDA/ISO Records | Documentation gaps, audit risk | Complete regulatory-ready records |
| Recall Readiness | Days to trace affected orders | 5-minute batch-to-customer report |
| Veeqo Integration | Manual reconciliation | Two-way sync, zero double-entry |
FAQ
Medical & Lab Supply Tracking Questions
Protect Patient Safety with Proper Expiry Management
Join medical and lab supply distributors using TraceLot to automate FEFO, stay compliant, and never ship expired products.
First month free. No credit card required.